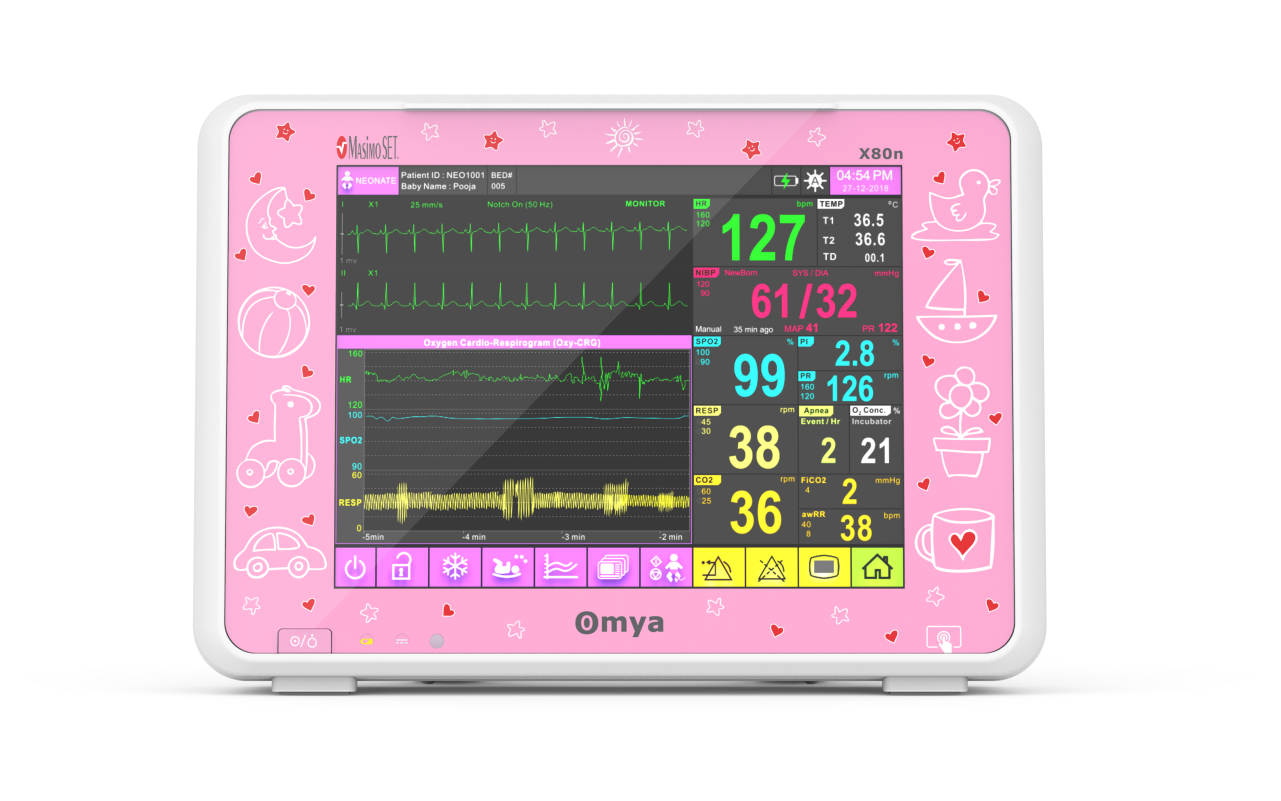
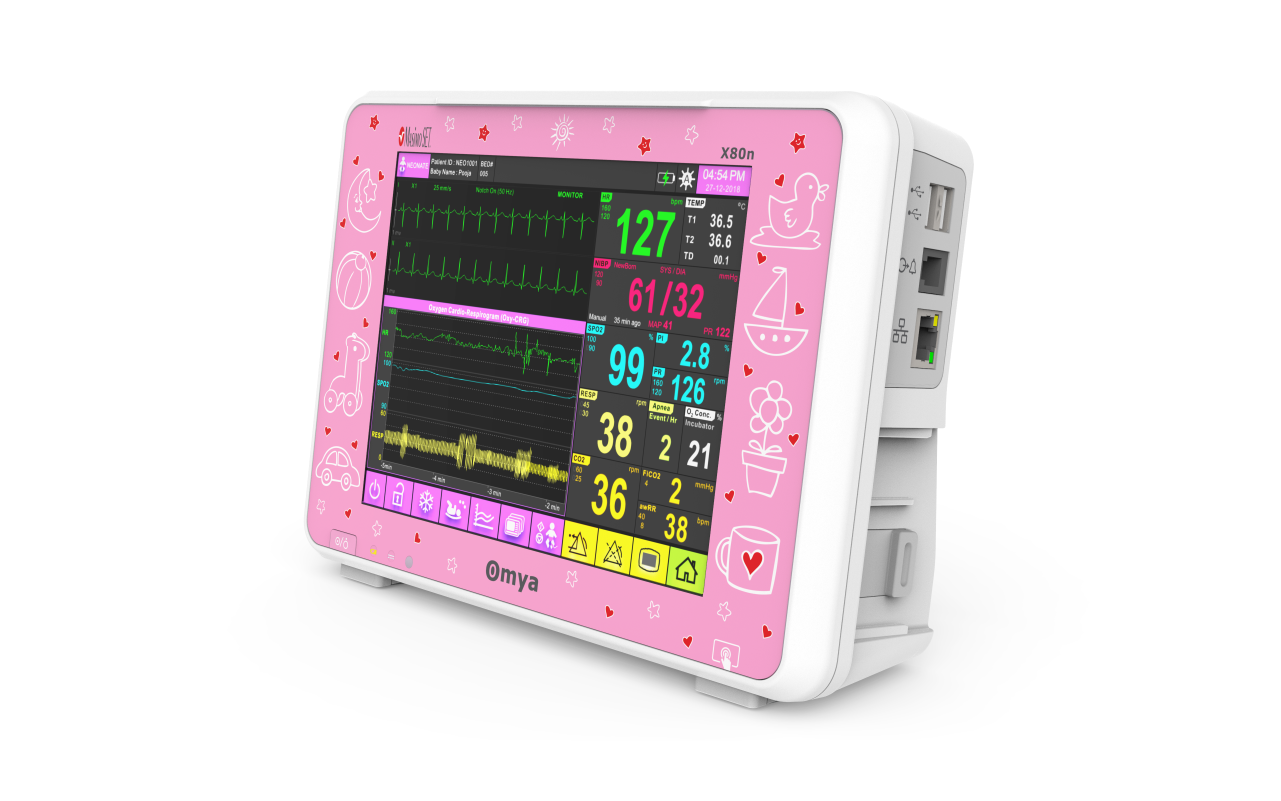
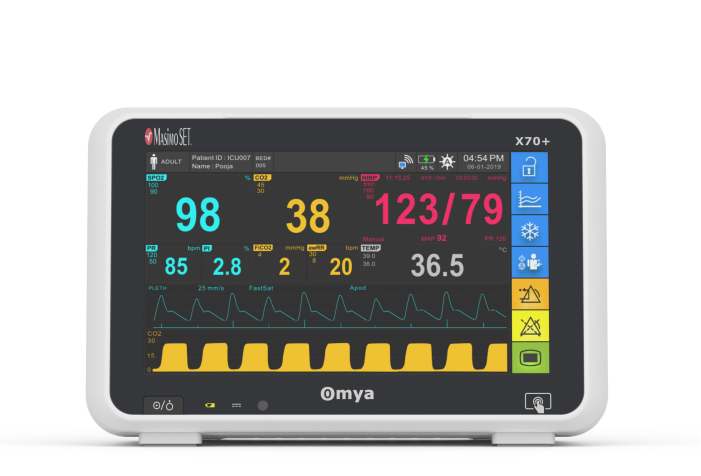
A compact ultra-thin lightweight design, Easy of use interface – choose either full touchscreen with intuitive menu system & simplify navigation provides the most-needed continuous vital sign parameters, Specialised wide-range of algorithms for accurate, easy, informed decision making. Completely valid and customizable alarms limits for the acuity area. available with semi-modular side-plug modules provides patient care flexibility.
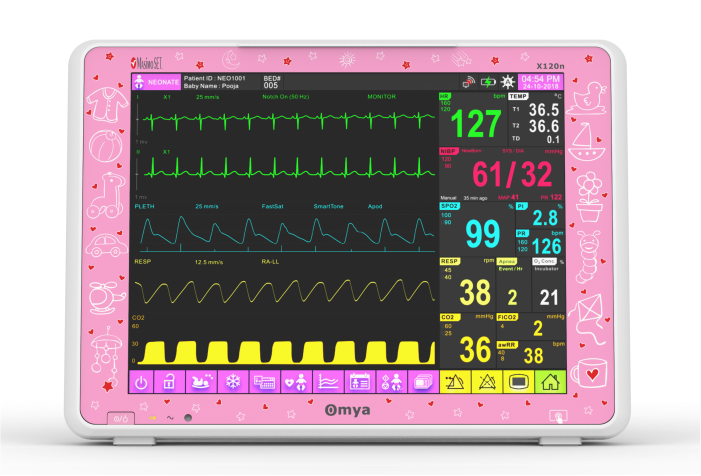
A care solution designed specifically for the Neonatal patient and their environment
Specialised wide-range of neonatal algorithms for accurate, easy, and informed decision making, Completely valid and customizable alarms limits for ICU use.
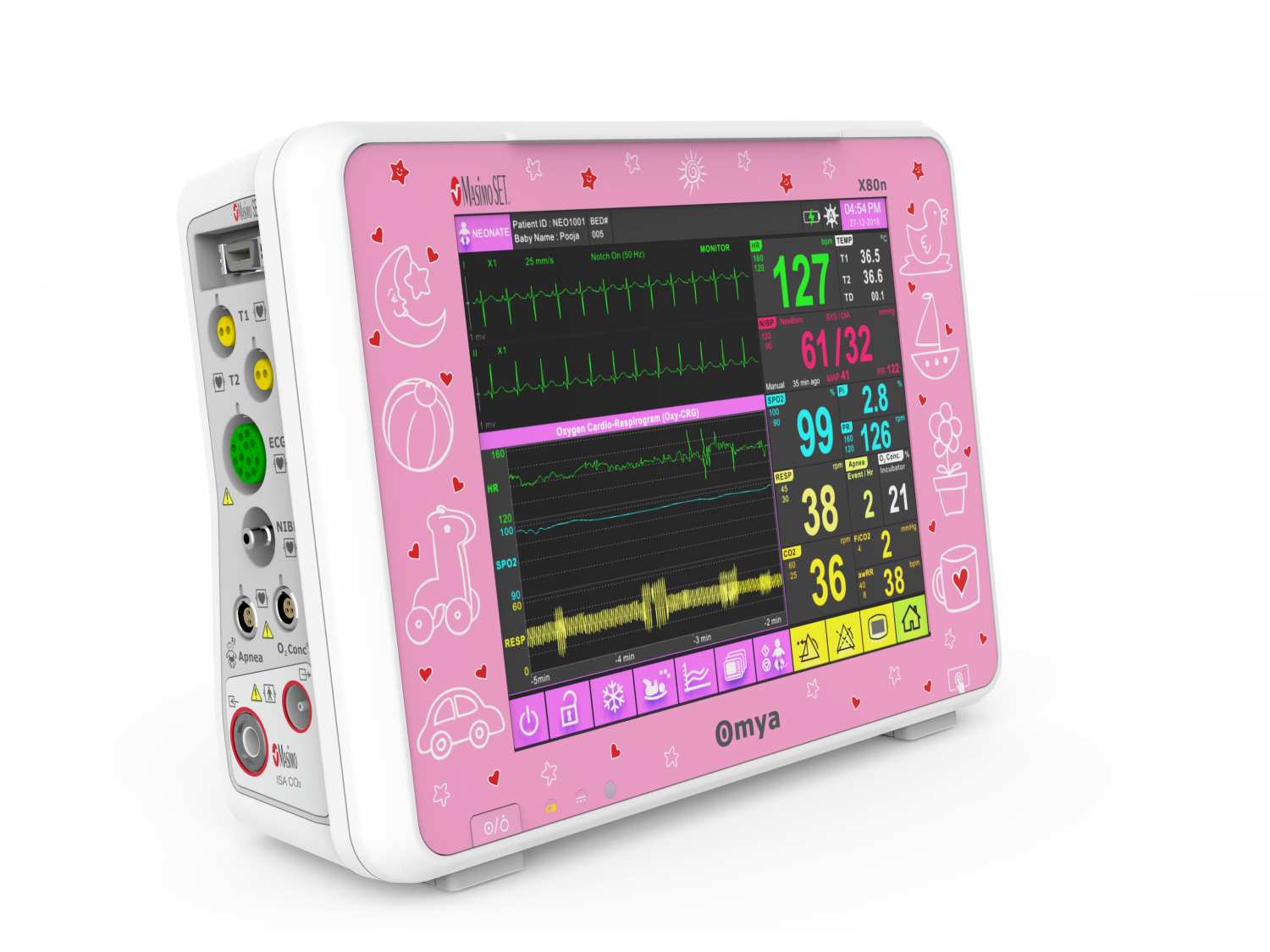
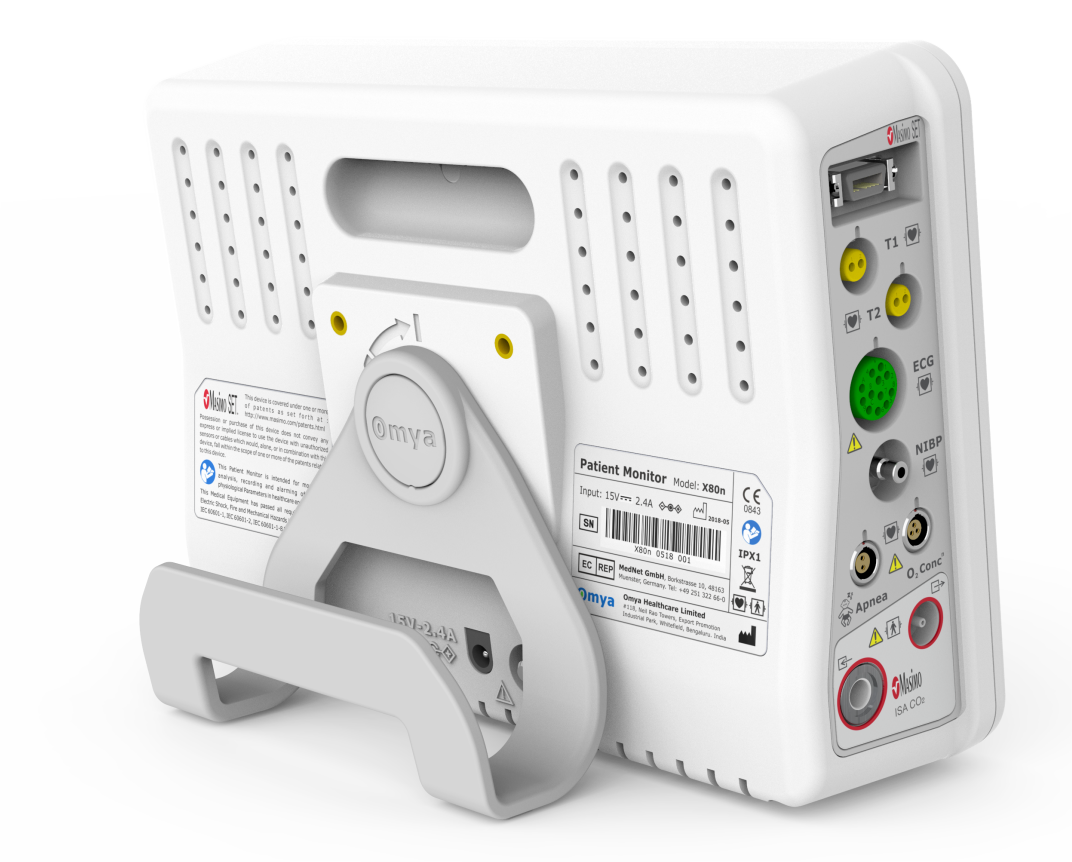
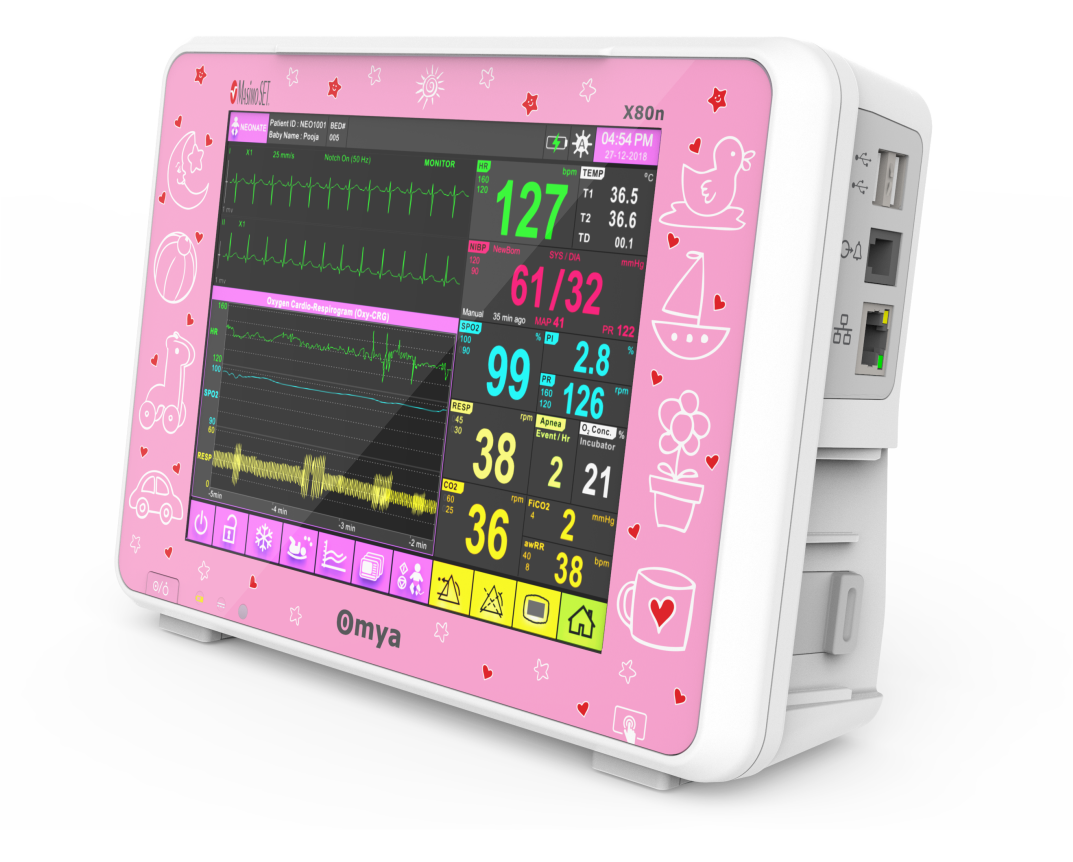

Technical Specifications
Parameter Specifications
ECG (Electrocardiogram) Specification
• Heart rate range
– Adult: 15 bpm to 300 bpm
– Pediatric and neonatal: 15 bpm to 350 bpm
• Heart rate accuracy: ± 1% or ± 1 bpm, whichever is greater
• Bandwidth
– Diagnostic Mode: 0.05Hz to 150Hz,
– Monitoring Mode: 0.5Hz to 55Hz
– Surgical mode: 1Hz to 20Hz
• Leads
– 3-lead, and 5-lead
• Display sweep speeds: 12.5, 25, and 50 mm/s
• Pacemaker detection: Indicator of pace pulse on
waveform display (user-selectable)
• ECG size (sensitivity): 4,0, 2.0, 1.0, 0.5, 0.25 cm/mV, or Auto
• Lead off condition detected and displayed
• Input signal range: ± 5 mV
Respiration
• Technique: Trans-thoracic impedance
• Measurement range: 3 rpm to 150 rpm
• Resolution: 1 rpm
• Accuracy:
– ± 1 rpm in the range 3 rpm to 120 rpm
– ± 2 rpm in the range 121 rpm to 150 rpm
• Respiration excitation waveform: < 250 μA, 37 kHz nominal
• ECG leads used: RA to LL
• Display sweep speeds: 6.25, 12.5, 25, 50 mm/s
• Lead off condition detected and displayed
Omya SpO2
• Measurement range
– SpO2: 0% to 100%
– SpO2 resolution: 1%
– Pulse rate: 30 bpm to 300 bpm
– Pulse rate resolution: 1 bpm
• Pulse rate accuracy: 2% or 1 bpm, whichever is greater
• SpO2 accuracyV (within the range 70% to 100%),
Philips reusable sensors
– ± 2% — M1191B, M1191BL, M1192A,
– ± 3% — M1193A, M1194A, M1195A, M1196A, M1191T, M1192T,
M1196T, M1196S
– ± 4% — M1193T (neonatal)
• SpO2 accuracyV (within the range 70% to 100%),
Philips disposable sensors
– ± 3% — M1131A, M1133A, M1134A (neonatal)
– ± 2% — M1132A, M1133A, M1134A (adult/infant)
• SpO2 accuracyV (within the range 70% to 100%),
Efficia sensors
– ± 3% — 989803160631, 989803160621, 989803160611
• Wavelength rangeVI: 500 nm to 1000 nm for all specified
sensors
• Maximum optical output power: ≤ 15 mW for all specified sensors
Non-invasive blood pressure (NIBP)
• Technique: Oscillometric, using stepwise deflation pressure
• Adult measurement range
– Systolic: 30 mmHg to 270 mmHg (4.0 kPa to 36.0 kPa)
– Diastolic: 10 mmHg to 245 mmHg (1.3 kPa to 32.7 kPa)
– MAP: 20 mmHg to 255 mmHg (2.7 kPa to 34.0 kPa)
• Pediatric measurement range
– Systolic: 30 mmHg to 180 mmHg (4.0 kPa to 24.0 kPa)
– Diastolic: 10 mmHg to 150 mmHg (1.3 kPa to 20.0 kPa)
– MAP: 20 mmHg to 160 mmHg (2.7 kPa to 21.3 kPa)
• Neonatal measurement range
– Systolic: 30 mmHg to 130 mmHg (4.0 kPa to 17.3 kPa)
– Diastolic: 10 mmHg to 100 mmHg (1.3 kPa to 13.3 kPa)
– MAP: 20 mmHg to 120 mmHg (2.7 kPa to 16.0 kPa)
• Blood pressure accuracy
– Maximum standard deviation: ≤ 8 mmHg
– Maximum mean error: ± 5 mmHg
• Pulse rate range: 40 bpm to 300 bpm
• Pulse rate accuracy (average over the NBP cycle)
– 40 bpm to 100 bpm: ± 5 bpm
– 101 bpm to 200 bpm: ± 5% of reading
– 201 bpm to 300 bpm: ± 10% of reading
• Initial cuff inflation
– Adult: 160 mmHg (21.3 kPa)
– Pediatric: 140 mmHg (18.7 kPa)
– Neonatal: 100 mmHg (13.3 kPa)
• NBP intervals: Automatic measurements at intervals of 1, 2, 3, 5,
10, 15, 30, 60, 90, 120 minutes, and STAT
Mainstream CO2 (EtCO2)
• Measurement range:
– 0 mmHg to 150 mmHgIX
• imCO2 measurement range (based on lowest reading over
last 20 seconds): 3 mmHg to 50 mmHg
• Data sample rate: Waveform sampling, 20 samples per second
• CO2 waveform resolution: 0.1 mmHg
• etCO2, imCO2 resolution: 1.0 mmHg
• Initialization time: Full specification etCO2 measurement
displays after warm up, in less than 2 minutes
• Total response time: < 2 seconds
• Calibration interval: No calibration required
• Auto zero interval: Only required when changing the airway
adapter style.
• Accuracy (gas temperature at 35°C):
– ± 2 mmHg in the range 0 to 40 mmHg
– ± 5% of reading in the range 41 to 70 mmHg
– ± 8% of reading in the range of 71 to 100 mmHg
– ± 10% of reading in the range of 101 to 150 mmHg
• Respiration rate range: 0 to 150 rpm
• Respiration rate accuracy: ± 1 rpm
• Drift of measurement accuracy:
– Short term drift (4 hours of use): Does not exceed 0.8 mmHg
– Long term drift (120 hour period): Retains accuracy specification
• Barometric pressure: Configured by system administrator
ECG arrhythmia
– Respiration excitation waveform: < 250 μA, 37 kHz nominal
– Time to alarm for tachycardia: < 5.0 seconds
– Tall T-wave rejection capability: Tested to a T-wave
amplitude of 1.8 mV
– Three different heart rate averaging methods are used:
• Normally, by averaging the 12 most recent R to R intervals.
• For runs of PVCs, up to 8 R to R intervals are averaged.
• If each of 3 consecutive R to R intervals is greater than
1200 ms (that is, rate less than 50 bpm, 80 bpm
for neonates), then the 4 most recent R to R intervals
are averaged.
– Response time of heart rate meter to change in heart rate (HR
change from 80 bpm to 120 bpm, or change from
80 bpm to 40 bpm): 10 seconds maximum
– Heart rate meter accuracy and response to irregular rhythm:
Ventricular bigeminy: 80 bpm
Slow alternating ventricular bigeminy: 60 bpm
Rapid alternating ventricular bigeminy: 120 bpm
Bidirectional systoles: 90 bpm
– Accuracy of input signal reproduction: Methods A and B
were used to establish overall system error and
frequency response
– Time to alarm for cardiac standstill: < 10 seconds
– Time to alarm for low heart rate: < 10 seconds
– Time to alarm for high heart rate: < 10 seconds
– Pacemaker pulse rejection: Rejects ± 2mV to ± 700 mV;
Pulse widths 0.1 to 2.0 ms; with no overshoot (Meets AAMI
EC13 using test method A)
– Pacer Pulse Detector Rejection of Fast ECG Signals:
With a 5mV input, a minimum slew rate of 1V/s. RTI will trigger
the pace pulse detector
Masimo SET® Module
• Sensors: Masimo SET® LNCS®
• Amplifier: Fully isolated, defibrillation protected > 5kV
• Sampling frequency: 62.5 Hz
• Display update interval: 1 second
• Signal IQ waveform: A waveform that indicates pulse detection confidence. Values range from 0 to 127 where 0 is low confidence and 127 is high confidence.
• Perfusion Index numeric (PI): A numeric provided that indicates perfusion. Perfusion is measured in % and ranges from 0.02% to 20%.
• Fast SAT: A mode that enables rapid tracking of arterial oxygen saturation changes by minimizing the averaging.
• Display ranges:
– Saturation (SpO2): 0 to 100%
– PR: 25 to 240 beats per minute
• Averaging Time: 2 / 4 / 8 / 10 / 12 / 14 / 16 / 18
• Sensitivity: Normal / Max / APOD
• Accuracy:
– SpO2, no motion: 60 to 80 ± 3%, adults / pediatrics
70 to 100 ± 2%, adults / pediatrics, ± 3% neonates
– SpO2, motion: 70 to 100 ± 3%, adults / pediatrics / neonates
– SpO2, low perfusion: 70 to 100 ± 2%, adults / pediatrics / neonates
– PR, no motion: 25 to 240 ± 3 bpm, adults / pediatrics / neonates
– PR, motion: 25 to 240 ± 5 bpm, adults / pediatrics / neonates
– PR, low perfusion: 25 to 240 ± 5 bpm, adults / pediatrics / neonates
Temperature measurements
• Measurement range for all measurement sites:
25°C to 45°C (77°F to 113°F)
• Probe accuracy
– ± 0.2°C — 21075A, 20176A, 21078A, 21091A, M1837A,
21096A, 21097A, M2255A
– ± 0.1°C — 21090A, 21093A, 21094A, 21095A
• Mode of operation: Direct mode
• Heating and cooling Transient response time: ≤ 150 sec
Invasive blood pressure
• Measurement range: -40 mmHg to 360 mmHg
• Input Sensitivity: 5 µV/V/mmHg
• Zero static offsets: Up to ± 200 mmHg with ± 1 mmHg accuracy
• Gain accuracy
– Accuracy: ± 1%
– Drift: less than 0.05%/°C
• Overall accuracy (including transducer):
± 4 mmHg or ± 4%, whichever is greater
• Volume displacement of CPJ840J6: 0.2 mm3/100 mmHg
• Warm up time of equipment and transducer: < 15 seconds
Cardiac output
• Measurement range
– Cardiac output: 0.00 L/min to 20.00 L/min
– Tblood: 27.0°C to 43.0°C
– Tinj: 0.00°C to 27°C
• Resolution
– Cardiac output: 0.01 L/min
– Tblood: 0.1°C
– Tinj: 0.1°C
– Tbc: 0.1°C
• Measurement accuracy
– Cardiac output: ± 5% or 0.2 L/min, whichever is greater,
for cardiac output ≤ 10 L/min; ± 8% for cardiac output > 10 L/min
– Tblood: ± 0.1°C
– Tinj: ± 0.1°C
• Response time
– Cardiac output: < 25 seconds after starting measurement
– Tblood: < 1 second
– Tinj: < 1 second
– Tblood curve: < 1 second
Device Application Specifications
Display
Display size:
8″ High-resolution Touchscreen display
Resolution 800 x 600 pixels
Frequency: 50/60 Hz
Viewing Angle: ± 15
Maximum number of waveform display:
7 traces
Display waveform:
ECG (up to 3 leads), Respiration, IBP,
SpO2, pulse wave, CO2,
Numerical data display:
Heart rate, VPC rate, ST level,
respiration rate, IBP (systolic, diastolic,
mean), NIBP (systolic, diastolic, MAP),
SpO2, pulse rate, temperature, CO,
cardiac index, injectate temperature,
blood temperature, O2 concentration,
FiCO2, EtCO2,
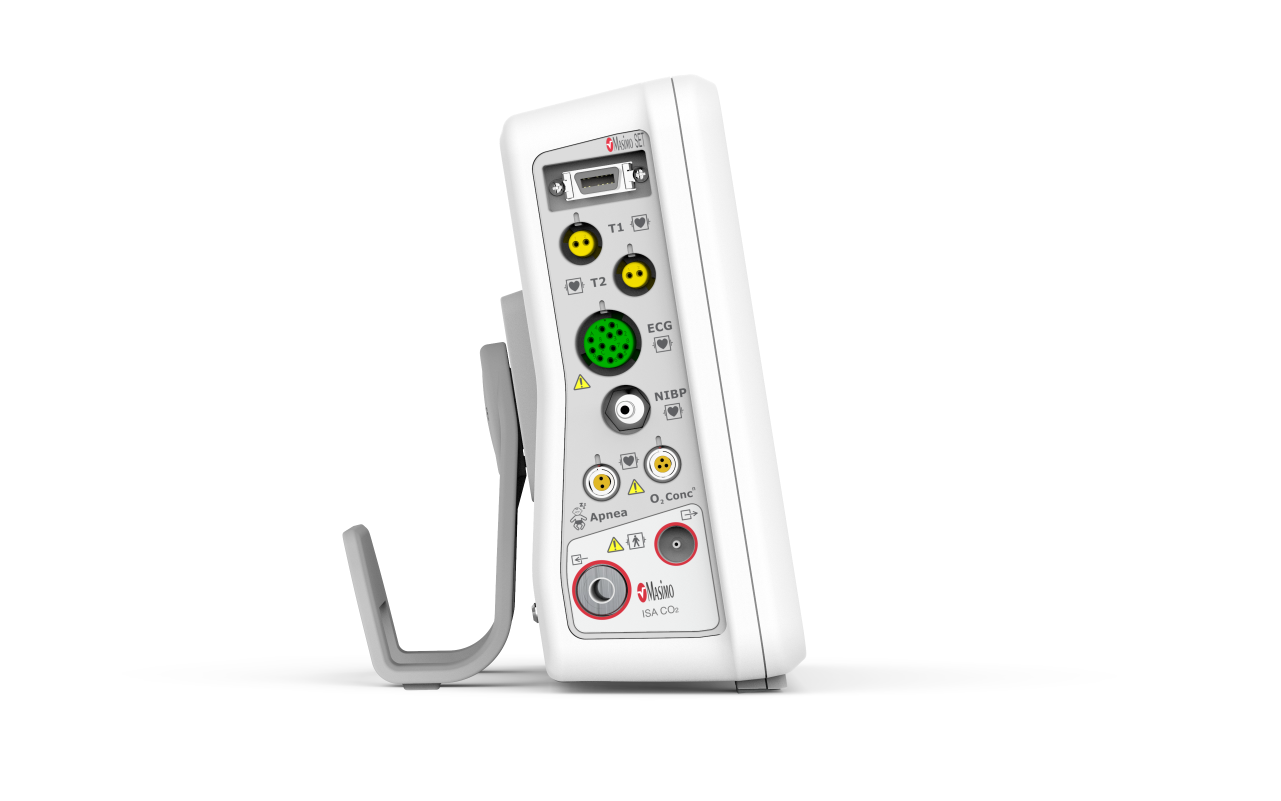
Communication Interfaces
• USB port (complies with the USB 2.0 standard
as a full-speed host), to
– Upgrade software
– Connect to a barcode scanner, or a serial
interface adapter
• Ethernet port, to
– Export HL7 data
– Connect the monitor to the Efficia CMS200 central station
• Wireless connectivityIII
Option E20 enables the monitor to access the EMR using
the customer’s existing wireless infrastructure. The monitor
supports the following wireless standards: IEEE802.11a, 802.11b,
802.11g, and 802.11n, operating in the 2.4 GHz or 5 GHz bands.
• EMR connectivity
– Via LAN
– Via WLAN
Power Supply
Integrated Power Supply,
Rated Voltage :
AC 100~240 V, 50~60 Hz,
Power Consumption :
25 VA
External DC power supply :
12V DC supply
Physical Dimensions
Dimension (L) x (W) x (H) in mm:
260 x 138 x 245
Weight :
3.7 kg (with clamp base)
Battery
Battery Capacity :
Advanced Rechargeable Lithium Polymer battery –
7.4 V, 4000 mAh (1 Internal battery)
Battery life :
Continuously operate for 6-8 hrs from Full Charge
(at a rate of 5 ml/h at 30°C under Normal Conditions)
Battery Recharge :
When AC Power is ON,
Battery will Automatically Recharge
(about 5 hrs to 90% charge ),
When the pump is off,
the charging time is not longer than 4 hrs
External Fuse
1.5 A / 250 V (User Replaceable)
Environmental Conditions
Operation Conditions:
Ambient temperature: + 5 to + 40°C,
Atmosphere Pressure: 86 ~106 kPa,
Relative humidity: ≤ 80%
Storage Conditions :
Ambient temperature: – 20 to +55°C,
Atmosphere Pressure: 50 ~106 kPa,
Relative humidity: ≤ 95%
Mechanical shock
Complies with mechanical shock requirement according to ISO 9919/IEC 80601-2-61 standards, for use within the healthcare facility.
Test conditions include:
• Peak acceleration: 150 m/s2 (15.3 g)
• Duration: 11 ms
• Pulse shape: Half sine
• Number of shocks: 3 shocks per direction per axis (18 total) Mechanical vibration
Complies with mechanical vibration requirement according to ISO 9919/IEC 80601-2-61 standards, for use within the healthcare facility.
Test conditions include:
• Frequency range: 10 Hz to 2000 Hz
• Resolution: 10 Hz
• Acceleration amplitude:
– 10 Hz to 100 Hz: 1.0 (m/s2)2/Hz
– 100 Hz to 200 Hz: -3.0 dB/octave
– 200 Hz to 2000 Hz: 0.5 (m/s2)2/Hz
• Duration: 10 minutes per each perpendicular axis (3 total)
Humidity
• Operating: 15% to 90% RH, non-condensing
• Storage: 15% to 90% RH, non-condensing
Safety Standards and Compliance
Classification & Electrical Safety Protection
Product Classification
According to the MDD 93/42/EEC Appendix Ⅸ Classification Rule 10: class II b
Type of protection against electric shock
Class I, equipment contains an internal powered equipment
Level of protection against electric shock
Application of CF-type non-defibrillation discharging effect protection
EMC type
Group 1, Class A
Anti-electroshock degree
ECG (RESP), TEMP, IBP, C.O., Quick Temp CF, SpO2, NIBP, CO2
Ingress Protection
IPx1 (No protection against ingress of water)
Disinfection/sterilization method
Standard method
Working system
Continuous operation equipment
Power supply way
Grid power plug, removable and soft wire power supply
Visual Alarm Indication
Visual Alarms indicates with two priority colors:
Red (High Priority) and
Yellow (Low and Mid Priority),
meets according to the IEC 60601-1-8 standard requirements.
Safety Standards
EN/IEC 60601-1
EN/IEC 60601-1-2
EN/IEC 60601-1-8
EN/IEC 60601-2-27
EN/ IEC 80601-2-30
IEC 60601-2-34
IEC 60601-2-49
EN/ISO 80601-2-55
EN/ISO 80601-2-61
EN/IEC 62366
EN/IEC 62304
EN/IEC 60601-1-6
EN/ISO 80601-2-56
• Conformity: CE according to directive 93/42/EEC class IIb
• Protection class: Protection against electric shocks, Class I, internally powered equipment, per EN/IEC 60601-1
• Degree of protection: Type CF defibrillator-proof, per EN/IEC 60601-1
• IPX1 Ingress protection against vertically falling water drops, This device is not designed for outdoor use
• Protection against hazards of ignition of flammable anesthetic mixtures:
Equipment is not suitable for use in the presence of a flammable anesthetic mixtures with air or oxygen or nitrous
oxide, per IEC 60601-1
Additional requirements: EN 1060-1, EN 1060-3 and EN 12470-4
Audio Alarm Volume :
Support multi-level volume functions
with the sound pressure range from 59-74 dB(A)
and key pad beep;
The alarm sound meets according to
the IEC 60601-1-8 standard requirements.
General Specifications
Display
Display size:
8″ High-resolution Touchscreen display
Resolution 800 x 600 pixels
Frequency: 50/60 Hz
Viewing Angle: ± 15
Maximum number of waveform display:
7 traces
Display waveform:
ECG (up to 3 leads), Respiration, IBP,
SpO2, pulse wave, CO2,
Numerical data display:
Heart rate, VPC rate, ST level,
respiration rate, IBP (systolic, diastolic,
mean), NIBP (systolic, diastolic, MAP),
SpO2, pulse rate, temperature, CO,
cardiac index, injectate temperature,
blood temperature, O2 concentration,
FiCO2, EtCO2,
Communication Interfaces
• USB port (complies with the USB 2.0 standard
as a full-speed host), to
– Upgrade software
– Connect to a barcode scanner, or a serial
interface adapter
• Ethernet port, to
– Export HL7 data
– Connect the monitor to the Efficia CMS200 central station
• Wireless connectivityIII
Option E20 enables the monitor to access the EMR using
the customer’s existing wireless infrastructure. The monitor
supports the following wireless standards: IEEE802.11a, 802.11b,
802.11g, and 802.11n, operating in the 2.4 GHz or 5 GHz bands.
• EMR connectivity
– Via LAN
– Via WLAN
Power Supply
Integrated Power Supply,
Rated Voltage :
AC 100~240 V, 50~60 Hz,
Power Consumption :
25 VA
External DC power supply :
12V DC supply
Physical Dimensions
Dimension (L) x (W) x (H) in mm:
260 x 138 x 245
Weight :
3.7 kg (with clamp base)
Battery
Battery Capacity :
Advanced Rechargeable Lithium Polymer battery –
7.4 V, 4000 mAh (1 Internal battery)
Battery life :
Continuously operate for 6-8 hrs from Full Charge
(at a rate of 5 ml/h at 30°C under Normal Conditions)
Battery Recharge :
When AC Power is ON,
Battery will Automatically Recharge
(about 5 hrs to 90% charge ),
When the pump is off,
the charging time is not longer than 4 hrs
External Fuse
1.5 A / 250 V (User Replaceable)
Environmental Conditions
Operation Conditions:
Ambient temperature: + 5 to + 40°C,
Atmosphere Pressure: 86 ~106 kPa,
Relative humidity: ≤ 80%
Storage Conditions :
Ambient temperature: – 20 to +55°C,
Atmosphere Pressure: 50 ~106 kPa,
Relative humidity: ≤ 95%
Mechanical shock
Complies with mechanical shock requirement according to ISO 9919/IEC 80601-2-61 standards, for use within the healthcare facility.
Test conditions include:
• Peak acceleration: 150 m/s2 (15.3 g)
• Duration: 11 ms
• Pulse shape: Half sine
• Number of shocks: 3 shocks per direction per axis (18 total) Mechanical vibration
Complies with mechanical vibration requirement according to ISO 9919/IEC 80601-2-61 standards, for use within the healthcare facility.
Test conditions include:
• Frequency range: 10 Hz to 2000 Hz
• Resolution: 10 Hz
• Acceleration amplitude:
– 10 Hz to 100 Hz: 1.0 (m/s2)2/Hz
– 100 Hz to 200 Hz: -3.0 dB/octave
– 200 Hz to 2000 Hz: 0.5 (m/s2)2/Hz
• Duration: 10 minutes per each perpendicular axis (3 total)
Humidity
• Operating: 15% to 90% RH, non-condensing
• Storage: 15% to 90% RH, non-condensing
Configuration and Optional Features
Event Log Review
Approximately one year event log of normal use and
Min. 2000 records with time and date
Mounting options
The monitor has the following mounting options:
• Roll stand
• Roll stand mounting kit
• Wall mount
• Wall channel
• Bedrail hook
Wireless LAN (Optional)
Wireless Data Transmission compatible with Patient Data Management Systems (Optional)
Nurse Staff call (Optional)
Max. 24 V / 0,5 A / 24 VA
- Parameter Specifications
-
Parameter Specifications
ECG (Electrocardiogram) Specification
• Heart rate range
– Adult: 15 bpm to 300 bpm
– Pediatric and neonatal: 15 bpm to 350 bpm
• Heart rate accuracy: ± 1% or ± 1 bpm, whichever is greater
• Bandwidth
– Diagnostic Mode: 0.05Hz to 150Hz,
– Monitoring Mode: 0.5Hz to 55Hz
– Surgical mode: 1Hz to 20Hz
• Leads
– 3-lead, and 5-lead
• Display sweep speeds: 12.5, 25, and 50 mm/s
• Pacemaker detection: Indicator of pace pulse on
waveform display (user-selectable)
• ECG size (sensitivity): 4,0, 2.0, 1.0, 0.5, 0.25 cm/mV, or Auto
• Lead off condition detected and displayed
• Input signal range: ± 5 mVRespiration
• Technique: Trans-thoracic impedance
• Measurement range: 3 rpm to 150 rpm
• Resolution: 1 rpm
• Accuracy:
– ± 1 rpm in the range 3 rpm to 120 rpm
– ± 2 rpm in the range 121 rpm to 150 rpm
• Respiration excitation waveform: < 250 μA, 37 kHz nominal
• ECG leads used: RA to LL
• Display sweep speeds: 6.25, 12.5, 25, 50 mm/s
• Lead off condition detected and displayedOmya SpO2
• Measurement range
– SpO2: 0% to 100%
– SpO2 resolution: 1%
– Pulse rate: 30 bpm to 300 bpm
– Pulse rate resolution: 1 bpm
• Pulse rate accuracy: 2% or 1 bpm, whichever is greater
• SpO2 accuracyV (within the range 70% to 100%),
Philips reusable sensors
– ± 2% — M1191B, M1191BL, M1192A,
– ± 3% — M1193A, M1194A, M1195A, M1196A, M1191T, M1192T,
M1196T, M1196S
– ± 4% — M1193T (neonatal)
• SpO2 accuracyV (within the range 70% to 100%),
Philips disposable sensors
– ± 3% — M1131A, M1133A, M1134A (neonatal)
– ± 2% — M1132A, M1133A, M1134A (adult/infant)
• SpO2 accuracyV (within the range 70% to 100%),
Efficia sensors
– ± 3% — 989803160631, 989803160621, 989803160611
• Wavelength rangeVI: 500 nm to 1000 nm for all specified
sensors
• Maximum optical output power: ≤ 15 mW for all specified sensorsNon-invasive blood pressure (NIBP)
• Technique: Oscillometric, using stepwise deflation pressure
• Adult measurement range
– Systolic: 30 mmHg to 270 mmHg (4.0 kPa to 36.0 kPa)
– Diastolic: 10 mmHg to 245 mmHg (1.3 kPa to 32.7 kPa)
– MAP: 20 mmHg to 255 mmHg (2.7 kPa to 34.0 kPa)
• Pediatric measurement range
– Systolic: 30 mmHg to 180 mmHg (4.0 kPa to 24.0 kPa)
– Diastolic: 10 mmHg to 150 mmHg (1.3 kPa to 20.0 kPa)
– MAP: 20 mmHg to 160 mmHg (2.7 kPa to 21.3 kPa)
• Neonatal measurement range
– Systolic: 30 mmHg to 130 mmHg (4.0 kPa to 17.3 kPa)
– Diastolic: 10 mmHg to 100 mmHg (1.3 kPa to 13.3 kPa)
– MAP: 20 mmHg to 120 mmHg (2.7 kPa to 16.0 kPa)
• Blood pressure accuracy
– Maximum standard deviation: ≤ 8 mmHg
– Maximum mean error: ± 5 mmHg
• Pulse rate range: 40 bpm to 300 bpm
• Pulse rate accuracy (average over the NBP cycle)
– 40 bpm to 100 bpm: ± 5 bpm
– 101 bpm to 200 bpm: ± 5% of reading
– 201 bpm to 300 bpm: ± 10% of reading
• Initial cuff inflation
– Adult: 160 mmHg (21.3 kPa)
– Pediatric: 140 mmHg (18.7 kPa)
– Neonatal: 100 mmHg (13.3 kPa)
• NBP intervals: Automatic measurements at intervals of 1, 2, 3, 5,
10, 15, 30, 60, 90, 120 minutes, and STATMainstream CO2 (EtCO2)
• Measurement range:
– 0 mmHg to 150 mmHgIX
• imCO2 measurement range (based on lowest reading over
last 20 seconds): 3 mmHg to 50 mmHg
• Data sample rate: Waveform sampling, 20 samples per second
• CO2 waveform resolution: 0.1 mmHg
• etCO2, imCO2 resolution: 1.0 mmHg
• Initialization time: Full specification etCO2 measurement
displays after warm up, in less than 2 minutes
• Total response time: < 2 seconds
• Calibration interval: No calibration required
• Auto zero interval: Only required when changing the airway
adapter style.
• Accuracy (gas temperature at 35°C):
– ± 2 mmHg in the range 0 to 40 mmHg
– ± 5% of reading in the range 41 to 70 mmHg
– ± 8% of reading in the range of 71 to 100 mmHg
– ± 10% of reading in the range of 101 to 150 mmHg
• Respiration rate range: 0 to 150 rpm
• Respiration rate accuracy: ± 1 rpm
• Drift of measurement accuracy:
– Short term drift (4 hours of use): Does not exceed 0.8 mmHg
– Long term drift (120 hour period): Retains accuracy specification
• Barometric pressure: Configured by system administratorECG arrhythmia
– Respiration excitation waveform: < 250 μA, 37 kHz nominal
– Time to alarm for tachycardia: < 5.0 seconds
– Tall T-wave rejection capability: Tested to a T-wave
amplitude of 1.8 mV
– Three different heart rate averaging methods are used:
• Normally, by averaging the 12 most recent R to R intervals.
• For runs of PVCs, up to 8 R to R intervals are averaged.
• If each of 3 consecutive R to R intervals is greater than
1200 ms (that is, rate less than 50 bpm, 80 bpm
for neonates), then the 4 most recent R to R intervals
are averaged.
– Response time of heart rate meter to change in heart rate (HR
change from 80 bpm to 120 bpm, or change from
80 bpm to 40 bpm): 10 seconds maximum
– Heart rate meter accuracy and response to irregular rhythm:
Ventricular bigeminy: 80 bpm
Slow alternating ventricular bigeminy: 60 bpm
Rapid alternating ventricular bigeminy: 120 bpm
Bidirectional systoles: 90 bpm
– Accuracy of input signal reproduction: Methods A and B
were used to establish overall system error and
frequency response
– Time to alarm for cardiac standstill: < 10 seconds
– Time to alarm for low heart rate: < 10 seconds
– Time to alarm for high heart rate: < 10 seconds
– Pacemaker pulse rejection: Rejects ± 2mV to ± 700 mV;
Pulse widths 0.1 to 2.0 ms; with no overshoot (Meets AAMI
EC13 using test method A)
– Pacer Pulse Detector Rejection of Fast ECG Signals:
With a 5mV input, a minimum slew rate of 1V/s. RTI will trigger
the pace pulse detectorMasimo SET® Module
• Sensors: Masimo SET® LNCS®
• Amplifier: Fully isolated, defibrillation protected > 5kV
• Sampling frequency: 62.5 Hz
• Display update interval: 1 second
• Signal IQ waveform: A waveform that indicates pulse detection confidence. Values range from 0 to 127 where 0 is low confidence and 127 is high confidence.
• Perfusion Index numeric (PI): A numeric provided that indicates perfusion. Perfusion is measured in % and ranges from 0.02% to 20%.
• Fast SAT: A mode that enables rapid tracking of arterial oxygen saturation changes by minimizing the averaging.
• Display ranges:
– Saturation (SpO2): 0 to 100%
– PR: 25 to 240 beats per minute
• Averaging Time: 2 / 4 / 8 / 10 / 12 / 14 / 16 / 18
• Sensitivity: Normal / Max / APOD
• Accuracy:
– SpO2, no motion: 60 to 80 ± 3%, adults / pediatrics
70 to 100 ± 2%, adults / pediatrics, ± 3% neonates
– SpO2, motion: 70 to 100 ± 3%, adults / pediatrics / neonates
– SpO2, low perfusion: 70 to 100 ± 2%, adults / pediatrics / neonates
– PR, no motion: 25 to 240 ± 3 bpm, adults / pediatrics / neonates
– PR, motion: 25 to 240 ± 5 bpm, adults / pediatrics / neonates
– PR, low perfusion: 25 to 240 ± 5 bpm, adults / pediatrics / neonatesTemperature measurements
• Measurement range for all measurement sites:
25°C to 45°C (77°F to 113°F)
• Probe accuracy
– ± 0.2°C — 21075A, 20176A, 21078A, 21091A, M1837A,
21096A, 21097A, M2255A
– ± 0.1°C — 21090A, 21093A, 21094A, 21095A
• Mode of operation: Direct mode
• Heating and cooling Transient response time: ≤ 150 secInvasive blood pressure
• Measurement range: -40 mmHg to 360 mmHg
• Input Sensitivity: 5 µV/V/mmHg
• Zero static offsets: Up to ± 200 mmHg with ± 1 mmHg accuracy
• Gain accuracy
– Accuracy: ± 1%
– Drift: less than 0.05%/°C
• Overall accuracy (including transducer):
± 4 mmHg or ± 4%, whichever is greater
• Volume displacement of CPJ840J6: 0.2 mm3/100 mmHg
• Warm up time of equipment and transducer: < 15 secondsCardiac output
• Measurement range
– Cardiac output: 0.00 L/min to 20.00 L/min
– Tblood: 27.0°C to 43.0°C
– Tinj: 0.00°C to 27°C
• Resolution
– Cardiac output: 0.01 L/min
– Tblood: 0.1°C
– Tinj: 0.1°C
– Tbc: 0.1°C
• Measurement accuracy
– Cardiac output: ± 5% or 0.2 L/min, whichever is greater,
for cardiac output ≤ 10 L/min; ± 8% for cardiac output > 10 L/min
– Tblood: ± 0.1°C
– Tinj: ± 0.1°C
• Response time
– Cardiac output: < 25 seconds after starting measurement
– Tblood: < 1 second
– Tinj: < 1 second
– Tblood curve: < 1 second - Application Specifications
-
Device Application Specifications
Display
Display size:
8″ High-resolution Touchscreen display
Resolution 800 x 600 pixels
Frequency: 50/60 Hz
Viewing Angle: ± 15Maximum number of waveform display:
7 tracesDisplay waveform:
ECG (up to 3 leads), Respiration, IBP,
SpO2, pulse wave, CO2,Numerical data display:
Heart rate, VPC rate, ST level,
respiration rate, IBP (systolic, diastolic,
mean), NIBP (systolic, diastolic, MAP),
SpO2, pulse rate, temperature, CO,
cardiac index, injectate temperature,
blood temperature, O2 concentration,
FiCO2, EtCO2,Communication Interfaces
• USB port (complies with the USB 2.0 standard
as a full-speed host), to
– Upgrade software
– Connect to a barcode scanner, or a serial
interface adapter
• Ethernet port, to
– Export HL7 data
– Connect the monitor to the Efficia CMS200 central station
• Wireless connectivityIII
Option E20 enables the monitor to access the EMR using
the customer’s existing wireless infrastructure. The monitor
supports the following wireless standards: IEEE802.11a, 802.11b,
802.11g, and 802.11n, operating in the 2.4 GHz or 5 GHz bands.
• EMR connectivity
– Via LAN
– Via WLANPower Supply
Integrated Power Supply,
Rated Voltage :
AC 100~240 V, 50~60 Hz,
Power Consumption :
25 VA
External DC power supply :
12V DC supplyPhysical Dimensions
Dimension (L) x (W) x (H) in mm:
260 x 138 x 245
Weight :
3.7 kg (with clamp base)Battery
Battery Capacity :
Advanced Rechargeable Lithium Polymer battery –
7.4 V, 4000 mAh (1 Internal battery)Battery life :
Continuously operate for 6-8 hrs from Full Charge
(at a rate of 5 ml/h at 30°C under Normal Conditions)Battery Recharge :
When AC Power is ON,
Battery will Automatically Recharge
(about 5 hrs to 90% charge ),
When the pump is off,
the charging time is not longer than 4 hrsExternal Fuse
1.5 A / 250 V (User Replaceable)
Environmental Conditions
Operation Conditions:
Ambient temperature: + 5 to + 40°C,
Atmosphere Pressure: 86 ~106 kPa,
Relative humidity: ≤ 80%Storage Conditions :
Ambient temperature: – 20 to +55°C,
Atmosphere Pressure: 50 ~106 kPa,
Relative humidity: ≤ 95%Mechanical shock
Complies with mechanical shock requirement according to ISO 9919/IEC 80601-2-61 standards, for use within the healthcare facility.
Test conditions include:
• Peak acceleration: 150 m/s2 (15.3 g)
• Duration: 11 ms
• Pulse shape: Half sine
• Number of shocks: 3 shocks per direction per axis (18 total) Mechanical vibration
Complies with mechanical vibration requirement according to ISO 9919/IEC 80601-2-61 standards, for use within the healthcare facility.
Test conditions include:
• Frequency range: 10 Hz to 2000 Hz
• Resolution: 10 Hz
• Acceleration amplitude:
– 10 Hz to 100 Hz: 1.0 (m/s2)2/Hz
– 100 Hz to 200 Hz: -3.0 dB/octave
– 200 Hz to 2000 Hz: 0.5 (m/s2)2/Hz
• Duration: 10 minutes per each perpendicular axis (3 total)
Humidity
• Operating: 15% to 90% RH, non-condensing
• Storage: 15% to 90% RH, non-condensing - Safety Standards and Compliance
-
Safety Standards and Compliance
Classification & Electrical Safety Protection
Product Classification
According to the MDD 93/42/EEC Appendix Ⅸ Classification Rule 10: class II bType of protection against electric shock
Class I, equipment contains an internal powered equipmentLevel of protection against electric shock
Application of CF-type non-defibrillation discharging effect protectionEMC type
Group 1, Class AAnti-electroshock degree
ECG (RESP), TEMP, IBP, C.O., Quick Temp CF, SpO2, NIBP, CO2Ingress Protection
IPx1 (No protection against ingress of water)Disinfection/sterilization method
Standard methodWorking system
Continuous operation equipmentPower supply way
Grid power plug, removable and soft wire power supplyVisual Alarm Indication
Visual Alarms indicates with two priority colors:
Red (High Priority) and
Yellow (Low and Mid Priority),
meets according to the IEC 60601-1-8 standard requirements.Safety Standards
EN/IEC 60601-1
EN/IEC 60601-1-2
EN/IEC 60601-1-8
EN/IEC 60601-2-27
EN/ IEC 80601-2-30
IEC 60601-2-34
IEC 60601-2-49
EN/ISO 80601-2-55
EN/ISO 80601-2-61
EN/IEC 62366
EN/IEC 62304
EN/IEC 60601-1-6
EN/ISO 80601-2-56• Conformity: CE according to directive 93/42/EEC class IIb
• Protection class: Protection against electric shocks, Class I, internally powered equipment, per EN/IEC 60601-1
• Degree of protection: Type CF defibrillator-proof, per EN/IEC 60601-1
• IPX1 Ingress protection against vertically falling water drops, This device is not designed for outdoor use
• Protection against hazards of ignition of flammable anesthetic mixtures:
Equipment is not suitable for use in the presence of a flammable anesthetic mixtures with air or oxygen or nitrous
oxide, per IEC 60601-1
Additional requirements: EN 1060-1, EN 1060-3 and EN 12470-4Audio Alarm Volume :
Support multi-level volume functions
with the sound pressure range from 59-74 dB(A)
and key pad beep;
The alarm sound meets according to
the IEC 60601-1-8 standard requirements. - General Specifications
-
General Specifications
Display
Display size:
8″ High-resolution Touchscreen display
Resolution 800 x 600 pixels
Frequency: 50/60 Hz
Viewing Angle: ± 15Maximum number of waveform display:
7 tracesDisplay waveform:
ECG (up to 3 leads), Respiration, IBP,
SpO2, pulse wave, CO2,Numerical data display:
Heart rate, VPC rate, ST level,
respiration rate, IBP (systolic, diastolic,
mean), NIBP (systolic, diastolic, MAP),
SpO2, pulse rate, temperature, CO,
cardiac index, injectate temperature,
blood temperature, O2 concentration,
FiCO2, EtCO2,Communication Interfaces
• USB port (complies with the USB 2.0 standard
as a full-speed host), to
– Upgrade software
– Connect to a barcode scanner, or a serial
interface adapter
• Ethernet port, to
– Export HL7 data
– Connect the monitor to the Efficia CMS200 central station
• Wireless connectivityIII
Option E20 enables the monitor to access the EMR using
the customer’s existing wireless infrastructure. The monitor
supports the following wireless standards: IEEE802.11a, 802.11b,
802.11g, and 802.11n, operating in the 2.4 GHz or 5 GHz bands.
• EMR connectivity
– Via LAN
– Via WLANPower Supply
Integrated Power Supply,
Rated Voltage :
AC 100~240 V, 50~60 Hz,
Power Consumption :
25 VA
External DC power supply :
12V DC supplyPhysical Dimensions
Dimension (L) x (W) x (H) in mm:
260 x 138 x 245
Weight :
3.7 kg (with clamp base)Battery
Battery Capacity :
Advanced Rechargeable Lithium Polymer battery –
7.4 V, 4000 mAh (1 Internal battery)Battery life :
Continuously operate for 6-8 hrs from Full Charge
(at a rate of 5 ml/h at 30°C under Normal Conditions)Battery Recharge :
When AC Power is ON,
Battery will Automatically Recharge
(about 5 hrs to 90% charge ),
When the pump is off,
the charging time is not longer than 4 hrsExternal Fuse
1.5 A / 250 V (User Replaceable)
Environmental Conditions
Operation Conditions:
Ambient temperature: + 5 to + 40°C,
Atmosphere Pressure: 86 ~106 kPa,
Relative humidity: ≤ 80%Storage Conditions :
Ambient temperature: – 20 to +55°C,
Atmosphere Pressure: 50 ~106 kPa,
Relative humidity: ≤ 95%Mechanical shock
Complies with mechanical shock requirement according to ISO 9919/IEC 80601-2-61 standards, for use within the healthcare facility.
Test conditions include:
• Peak acceleration: 150 m/s2 (15.3 g)
• Duration: 11 ms
• Pulse shape: Half sine
• Number of shocks: 3 shocks per direction per axis (18 total) Mechanical vibration
Complies with mechanical vibration requirement according to ISO 9919/IEC 80601-2-61 standards, for use within the healthcare facility.
Test conditions include:
• Frequency range: 10 Hz to 2000 Hz
• Resolution: 10 Hz
• Acceleration amplitude:
– 10 Hz to 100 Hz: 1.0 (m/s2)2/Hz
– 100 Hz to 200 Hz: -3.0 dB/octave
– 200 Hz to 2000 Hz: 0.5 (m/s2)2/Hz
• Duration: 10 minutes per each perpendicular axis (3 total)
Humidity
• Operating: 15% to 90% RH, non-condensing
• Storage: 15% to 90% RH, non-condensing - Configuration & Optional Features
-
Configuration and Optional Features
Event Log Review
Approximately one year event log of normal use and
Min. 2000 records with time and dateMounting options
The monitor has the following mounting options:
• Roll stand
• Roll stand mounting kit
• Wall mount
• Wall channel
• Bedrail hookWireless LAN (Optional)
Wireless Data Transmission compatible with Patient Data Management Systems (Optional)
Nurse Staff call (Optional)
Max. 24 V / 0,5 A / 24 VA
Related Products
Request a quick quote
If you have request of our products or you are interested in any other medical products,
please leave messages below and we’ll reply you soon as possible within 24 hours.