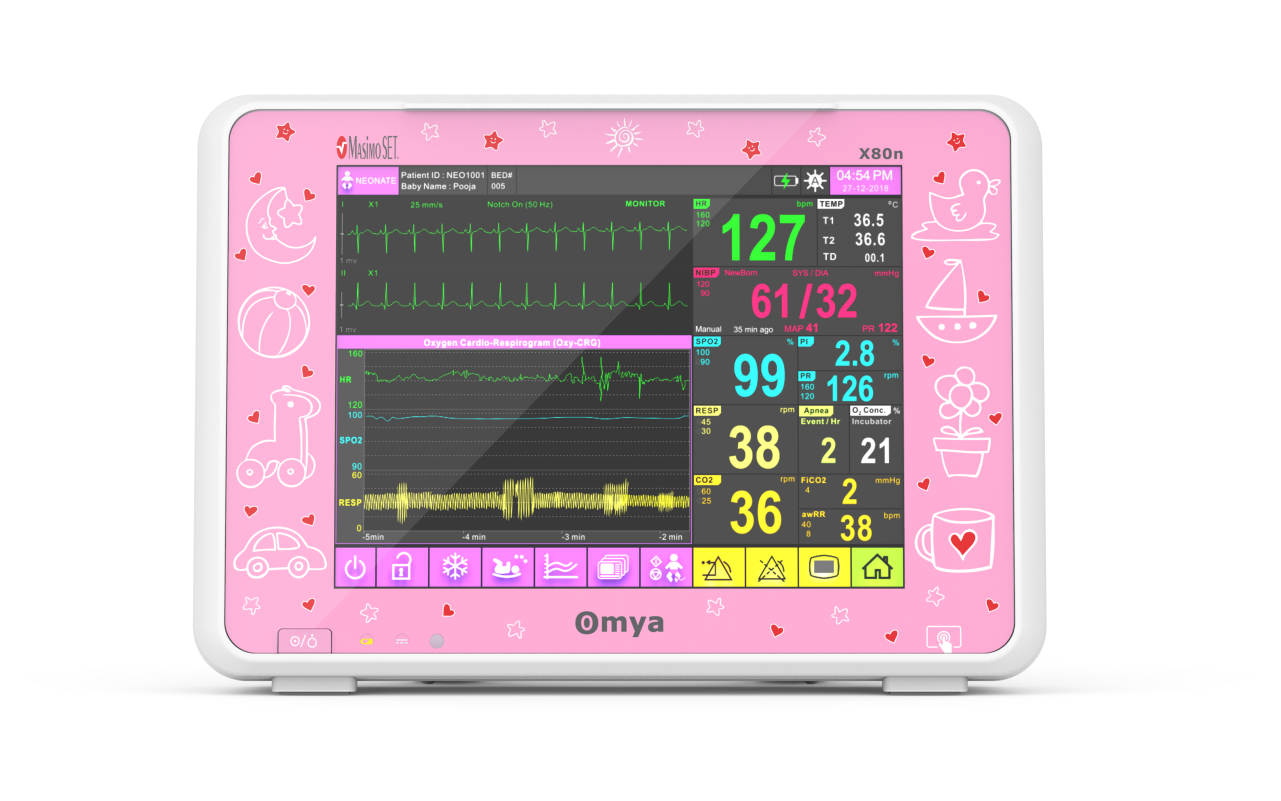
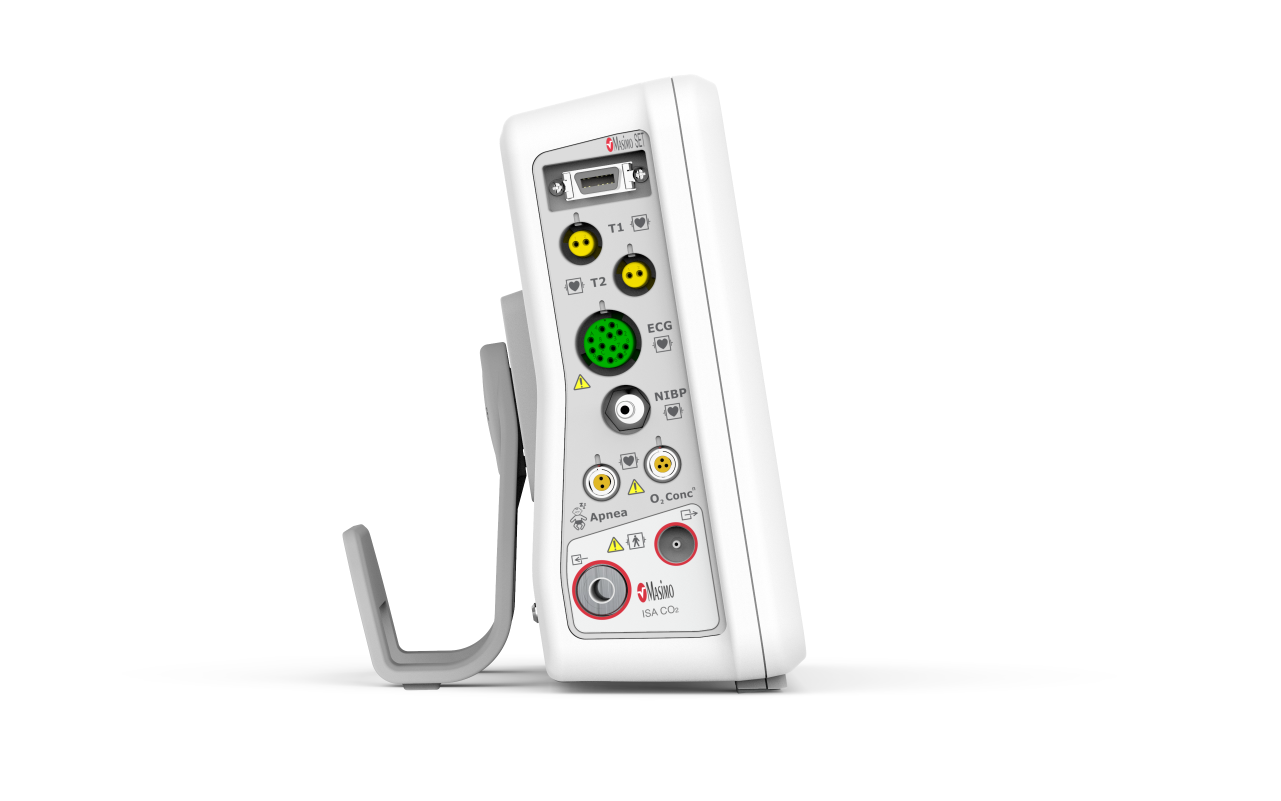
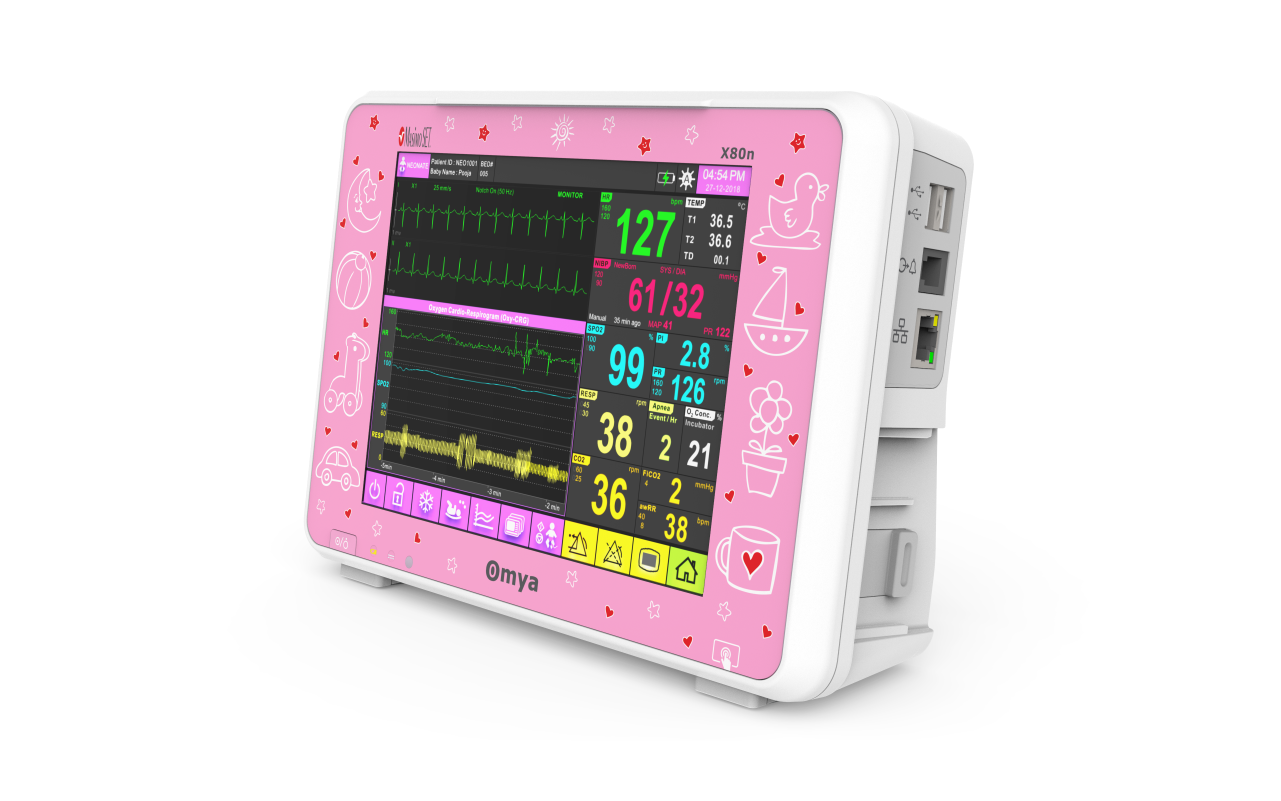
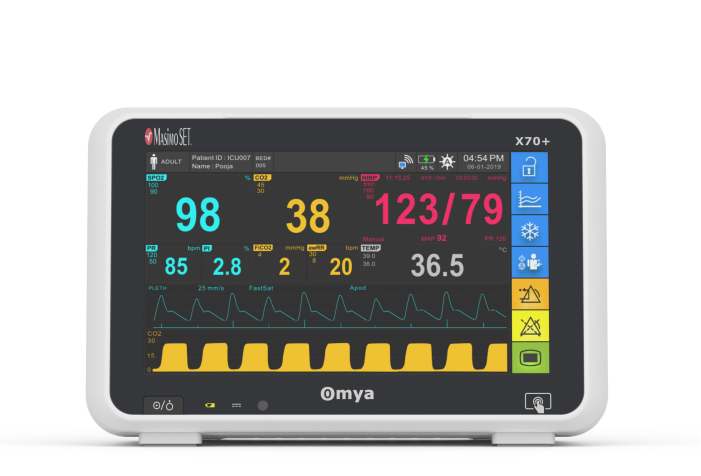
Omya’s Hand-Held Capnograph & Pulse Oximeter with Masimo Technology is an exceptionally comprehensive and versatile device intended for both spot checks and continuous monitoring. This hand-held portable monitor facilitates simultaneous end-tidal CO2 and SpO2 measurements is a variety of medical settings, including EMS/ED, transport, critical care, operating room, sleep lab and for procedural sedation.
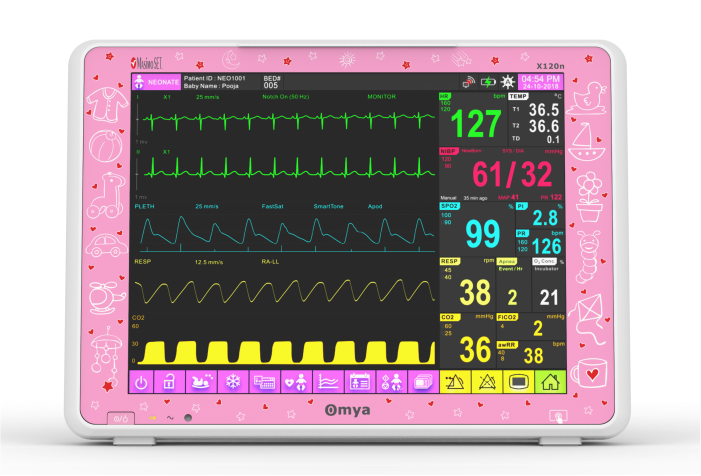
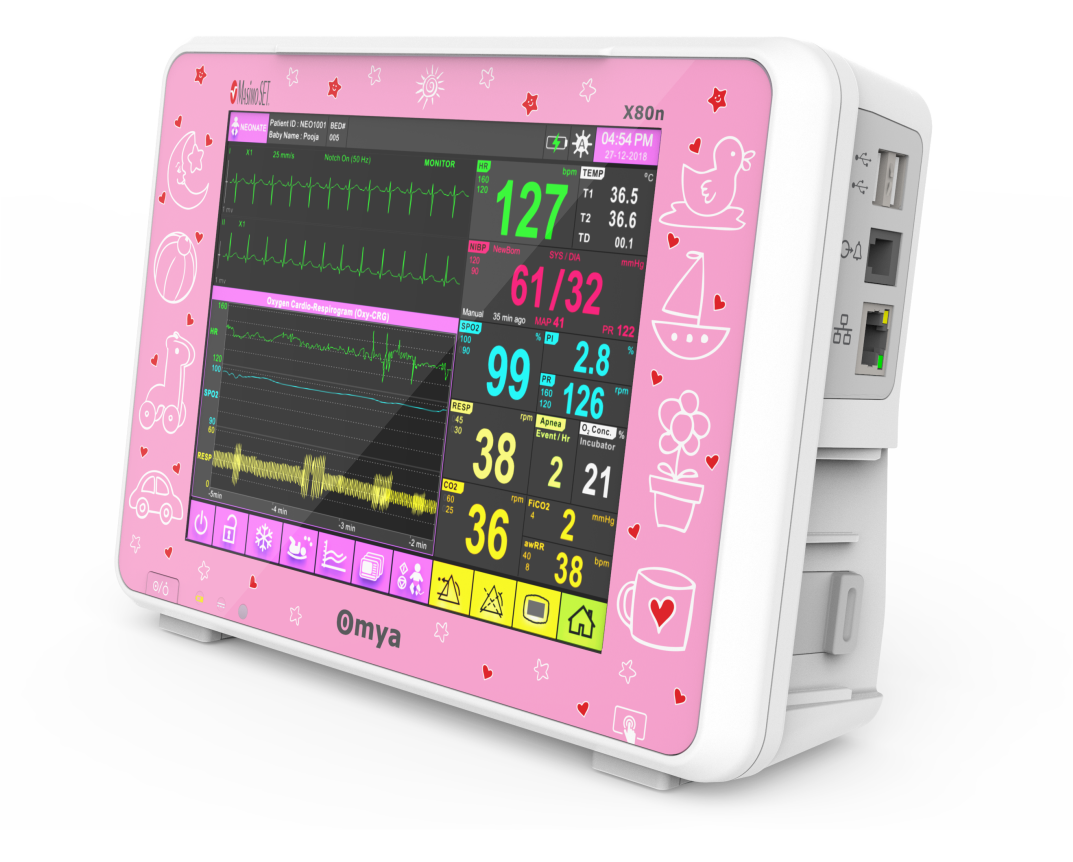
A care solution designed specifically for the Neonatal patient and their environment
Specialised wide-range of neonatal algorithms for accurate, easy, and informed decision making, Completely valid and customizable alarms limits for ICU use.


Technical Specifications
Parameter Specifications
Omya Mainstream CO2 (EtCO2)
• Measurement range:
– 0 mmHg to 150 mmHgIX
• imCO2 measurement range (based on lowest reading over
last 20 seconds): 3 mmHg to 50 mmHg
• Data sample rate: Waveform sampling, 20 samples per second
• CO2 waveform resolution: 0.1 mmHg
• etCO2, imCO2 resolution: 1.0 mmHg
• Initialization time: Full specification etCO2 measurement
displays after warm up, in less than 2 minutes
• Total response time: < 2 seconds
• Calibration interval: No calibration required
• Auto zero interval: Only required when changing the airway
adapter style.
• Accuracy (gas temperature at 35°C):
– ± 2 mmHg in the range 0 to 40 mmHg
– ± 5% of reading in the range 41 to 70 mmHg
– ± 8% of reading in the range of 71 to 100 mmHg
– ± 10% of reading in the range of 101 to 150 mmHg
• Respiration rate range: 0 to 150 rpm
• Respiration rate accuracy: ± 1 rpm
• Drift of measurement accuracy:
– Short term drift (4 hours of use): Does not exceed 0.8 mmHg
– Long term drift (120 hour period): Retains accuracy specification
• Barometric pressure: Configured by system administrator
Omya SpO2
• Measurement range
– SpO2: 0% to 100%
– SpO2 resolution: 1%
– Pulse rate: 30 bpm to 300 bpm
– Pulse rate resolution: 1 bpm
• Pulse rate accuracy: 2% or 1 bpm, whichever is greater
• SpO2 accuracyV (within the range 70% to 100%),
Philips reusable sensors
– ± 2% — M1191B, M1191BL, M1192A,
– ± 3% — M1193A, M1194A, M1195A, M1196A, M1191T, M1192T,
M1196T, M1196S
– ± 4% — M1193T (neonatal)
• SpO2 accuracyV (within the range 70% to 100%),
Philips disposable sensors
– ± 3% — M1131A, M1133A, M1134A (neonatal)
– ± 2% — M1132A, M1133A, M1134A (adult/infant)
• SpO2 accuracyV (within the range 70% to 100%),
Efficia sensors
– ± 3% — 989803160631, 989803160621, 989803160611
• Wavelength rangeVI: 500 nm to 1000 nm for all specified
sensors
• Maximum optical output power: ≤ 15 mW for all specified sensors
Device Application Specifications
Display
Display size:
4.3 inch High-resolution Touchscreen display
Resolution 480 x 272 pixels
Frequency: 50/60 Hz
Viewing Angle: ± 15
Display Screen:
Waveforms,numeric values,on-screen trend data.
Display waveform:
SpO2, Respiration, EtCO2
Numerical data display:
SpO2, pulse rate, temperature, O2 concentration,
FiCO2, EtCO2,
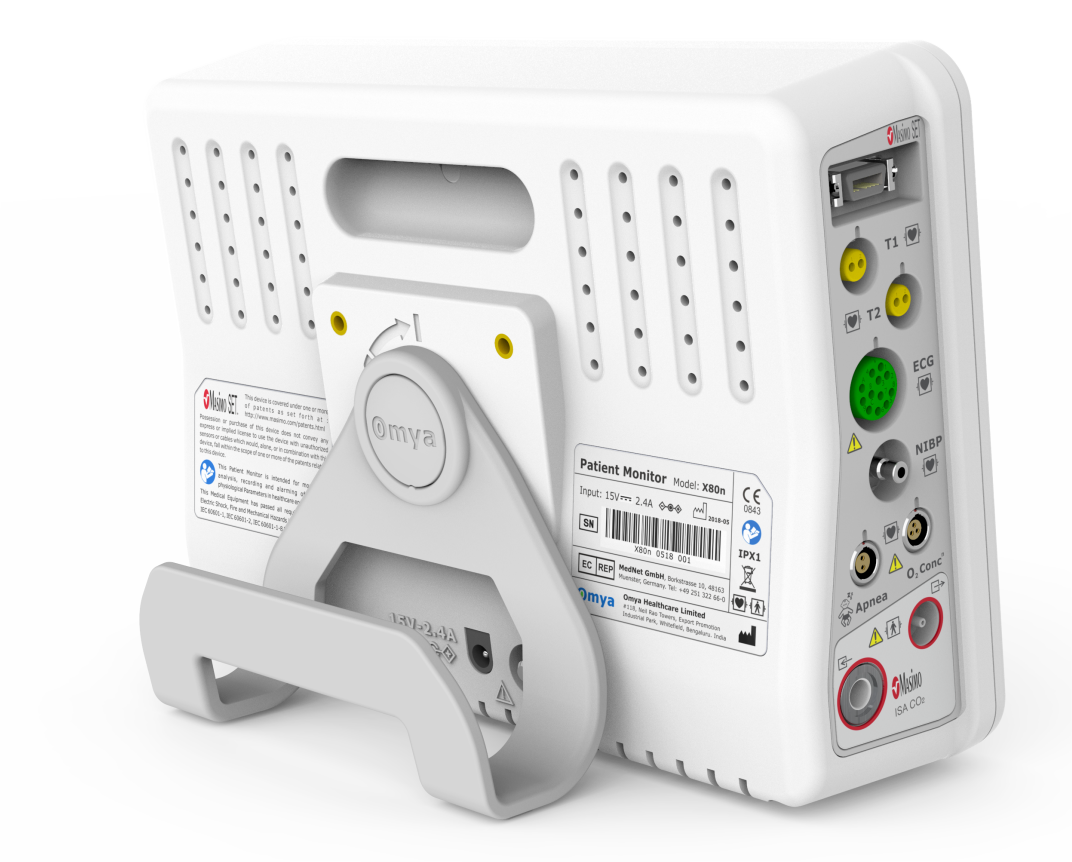
Communication Interfaces
• USB port (complies with the USB 2.0 standard
as a full-speed host), to
– Upgrade software
– Connect to a barcode scanner, or a serial
interface adapter
• Ethernet port, to
– Export HL7 data
– Connect the monitor to the Efficia CMS200 central station
• Wireless connectivityIII
Option E20 enables the monitor to access the EMR using
the customer’s existing wireless infrastructure. The monitor
supports the following wireless standards: IEEE802.11a, 802.11b,
802.11g, and 802.11n, operating in the 2.4 GHz or 5 GHz bands.
• EMR connectivity
– Via LAN
– Via WLAN
Power Supply
Integrated Power Supply,
Rated Voltage :
AC 100~240 V, 50~60 Hz,
Power Consumption :
25 VA
External DC power supply :
12V DC supply
Physical Dimensions
Dimension (H) x (W) x (T) in mm:
152 x 82 x 30.5 mm
Weight :
450g (with clamp base)
Battery
Battery Capacity :
Advanced Rechargeable Lithium Polymer battery –
7.4 V, 4000 mAh (1 Internal battery)
Battery life :
Continuously operate for 6-8 hrs from Full Charge
(at a rate of 5 ml/h at 30°C under Normal Conditions)
Battery Recharge :
When AC Power is ON,
Battery will Automatically Recharge
(about 5 hrs to 90% charge ),
When the pump is off,
the charging time is not longer than 4 hrs
External Fuse
1.5 A / 250 V (User Replaceable)
Environmental Conditions
Operation Conditions:
Ambient temperature: + 5 to + 40°C,
Atmosphere Pressure: 86 ~106 kPa,
Relative humidity: ≤ 80%
Storage Conditions :
Ambient temperature: – 20 to +55°C,
Atmosphere Pressure: 50 ~106 kPa,
Relative humidity: ≤ 95%
Mechanical shock
Complies with mechanical shock requirement according to ISO 9919/IEC 80601-2-61 standards, for use within the healthcare facility.
Test conditions include:
• Peak acceleration: 150 m/s2 (15.3 g)
• Duration: 11 ms
• Pulse shape: Half sine
• Number of shocks: 3 shocks per direction per axis (18 total) Mechanical vibration
Complies with mechanical vibration requirement according to ISO 9919/IEC 80601-2-61 standards, for use within the healthcare facility.
Test conditions include:
• Frequency range: 10 Hz to 2000 Hz
• Resolution: 10 Hz
• Acceleration amplitude:
– 10 Hz to 100 Hz: 1.0 (m/s2)2/Hz
– 100 Hz to 200 Hz: -3.0 dB/octave
– 200 Hz to 2000 Hz: 0.5 (m/s2)2/Hz
• Duration: 10 minutes per each perpendicular axis (3 total)
Humidity
• Operating: 15% to 90% RH, non-condensing
• Storage: 15% to 90% RH, non-condensing
Safety Standards and Compliance
Classification & Electrical Safety Protection
Product Classification
According to the MDD 93/42/EEC Appendix Ⅸ Classification Rule 10: class II b
Type of protection against electric shock
Class I, equipment contains an internal powered equipment
Level of protection against electric shock
Application of CF-type non-defibrillation discharging effect protection
EMC type
Group 1, Class A
Anti-electroshock degree
ECG (RESP), TEMP, IBP, C.O., Quick Temp CF, SpO2, NIBP, CO2
Ingress Protection
IPx1 (No protection against ingress of water)
Disinfection/sterilization method
Standard method
Working system
Continuous operation equipment
Power supply way
Grid power plug, removable and soft wire power supply
Visual Alarm Indication
Visual Alarms indicates with two priority colors:
Red (High Priority) and
Yellow (Low and Mid Priority),
meets according to the IEC 60601-1-8 standard requirements.
Safety Standards
EN/IEC 60601-1
EN/IEC 60601-1-2
EN/IEC 60601-1-8
EN/IEC 60601-2-27
EN/ IEC 80601-2-30
IEC 60601-2-34
IEC 60601-2-49
EN/ISO 80601-2-55
EN/ISO 80601-2-61
EN/IEC 62366
EN/IEC 62304
EN/IEC 60601-1-6
EN/ISO 80601-2-56
• Conformity: CE according to directive 93/42/EEC class IIb
• Protection class: Protection against electric shocks, Class I, internally powered equipment, per EN/IEC 60601-1
• Degree of protection: Type CF defibrillator-proof, per EN/IEC 60601-1
• IPX1 Ingress protection against vertically falling water drops, This device is not designed for outdoor use
• Protection against hazards of ignition of flammable anesthetic mixtures:
Equipment is not suitable for use in the presence of a flammable anesthetic mixtures with air or oxygen or nitrous
oxide, per IEC 60601-1
Additional requirements: EN 1060-1, EN 1060-3 and EN 12470-4
Audio Alarm Volume :
Support multi-level volume functions
with the sound pressure range from 59-74 dB(A)
and key pad beep;
The alarm sound meets according to
the IEC 60601-1-8 standard requirements.
General Specifications
Display
Display size:
8″ High-resolution Touchscreen display
Resolution 800 x 600 pixels
Frequency: 50/60 Hz
Viewing Angle: ± 15
Maximum number of waveform display:
7 traces
Display waveform:
ECG (up to 3 leads), Respiration, IBP,
SpO2, pulse wave, CO2,
Numerical data display:
Heart rate, VPC rate, ST level,
respiration rate, IBP (systolic, diastolic,
mean), NIBP (systolic, diastolic, MAP),
SpO2, pulse rate, temperature, CO,
cardiac index, injectate temperature,
blood temperature, O2 concentration,
FiCO2, EtCO2,
Communication Interfaces
• USB port (complies with the USB 2.0 standard
as a full-speed host), to
– Upgrade software
– Connect to a barcode scanner, or a serial
interface adapter
• Ethernet port, to
– Export HL7 data
– Connect the monitor to the Efficia CMS200 central station
• Wireless connectivityIII
Option E20 enables the monitor to access the EMR using
the customer’s existing wireless infrastructure. The monitor
supports the following wireless standards: IEEE802.11a, 802.11b,
802.11g, and 802.11n, operating in the 2.4 GHz or 5 GHz bands.
• EMR connectivity
– Via LAN
– Via WLAN
Power Supply
Integrated Power Supply,
Rated Voltage :
AC 100~240 V, 50~60 Hz,
Power Consumption :
25 VA
External DC power supply :
12V DC supply
Physical Dimensions
Dimension (L) x (W) x (H) in mm:
260 x 138 x 245
Weight :
3.7 kg (with clamp base)
Battery
Battery Capacity :
Advanced Rechargeable Lithium Polymer battery –
7.4 V, 4000 mAh (1 Internal battery)
Battery life :
Continuously operate for 6-8 hrs from Full Charge
(at a rate of 5 ml/h at 30°C under Normal Conditions)
Battery Recharge :
When AC Power is ON,
Battery will Automatically Recharge
(about 5 hrs to 90% charge ),
When the pump is off,
the charging time is not longer than 4 hrs
External Fuse
1.5 A / 250 V (User Replaceable)
Environmental Conditions
Operation Conditions:
Ambient temperature: + 5 to + 40°C,
Atmosphere Pressure: 86 ~106 kPa,
Relative humidity: ≤ 80%
Storage Conditions :
Ambient temperature: – 20 to +55°C,
Atmosphere Pressure: 50 ~106 kPa,
Relative humidity: ≤ 95%
Mechanical shock
Complies with mechanical shock requirement according to ISO 9919/IEC 80601-2-61 standards, for use within the healthcare facility.
Test conditions include:
• Peak acceleration: 150 m/s2 (15.3 g)
• Duration: 11 ms
• Pulse shape: Half sine
• Number of shocks: 3 shocks per direction per axis (18 total) Mechanical vibration
Complies with mechanical vibration requirement according to ISO 9919/IEC 80601-2-61 standards, for use within the healthcare facility.
Test conditions include:
• Frequency range: 10 Hz to 2000 Hz
• Resolution: 10 Hz
• Acceleration amplitude:
– 10 Hz to 100 Hz: 1.0 (m/s2)2/Hz
– 100 Hz to 200 Hz: -3.0 dB/octave
– 200 Hz to 2000 Hz: 0.5 (m/s2)2/Hz
• Duration: 10 minutes per each perpendicular axis (3 total)
Humidity
• Operating: 15% to 90% RH, non-condensing
• Storage: 15% to 90% RH, non-condensing
Configuration and Optional Features
Event Log Review
Approximately one year event log of normal use and
Min. 2000 records with time and date
Mounting options
The monitor has the following mounting options:
• Roll stand
• Roll stand mounting kit
• Wall mount
• Wall channel
• Bedrail hook
Wireless LAN (Optional)
Wireless Data Transmission compatible with Patient Data Management Systems (Optional)
Nurse Staff call (Optional)
Max. 24 V / 0,5 A / 24 VA
- Parameter Specifications
-
Parameter Specifications
Omya Mainstream CO2 (EtCO2)
• Measurement range:
– 0 mmHg to 150 mmHgIX
• imCO2 measurement range (based on lowest reading over
last 20 seconds): 3 mmHg to 50 mmHg
• Data sample rate: Waveform sampling, 20 samples per second
• CO2 waveform resolution: 0.1 mmHg
• etCO2, imCO2 resolution: 1.0 mmHg
• Initialization time: Full specification etCO2 measurement
displays after warm up, in less than 2 minutes
• Total response time: < 2 seconds
• Calibration interval: No calibration required
• Auto zero interval: Only required when changing the airway
adapter style.
• Accuracy (gas temperature at 35°C):
– ± 2 mmHg in the range 0 to 40 mmHg
– ± 5% of reading in the range 41 to 70 mmHg
– ± 8% of reading in the range of 71 to 100 mmHg
– ± 10% of reading in the range of 101 to 150 mmHg
• Respiration rate range: 0 to 150 rpm
• Respiration rate accuracy: ± 1 rpm
• Drift of measurement accuracy:
– Short term drift (4 hours of use): Does not exceed 0.8 mmHg
– Long term drift (120 hour period): Retains accuracy specification
• Barometric pressure: Configured by system administratorOmya SpO2
• Measurement range
– SpO2: 0% to 100%
– SpO2 resolution: 1%
– Pulse rate: 30 bpm to 300 bpm
– Pulse rate resolution: 1 bpm
• Pulse rate accuracy: 2% or 1 bpm, whichever is greater
• SpO2 accuracyV (within the range 70% to 100%),
Philips reusable sensors
– ± 2% — M1191B, M1191BL, M1192A,
– ± 3% — M1193A, M1194A, M1195A, M1196A, M1191T, M1192T,
M1196T, M1196S
– ± 4% — M1193T (neonatal)
• SpO2 accuracyV (within the range 70% to 100%),
Philips disposable sensors
– ± 3% — M1131A, M1133A, M1134A (neonatal)
– ± 2% — M1132A, M1133A, M1134A (adult/infant)
• SpO2 accuracyV (within the range 70% to 100%),
Efficia sensors
– ± 3% — 989803160631, 989803160621, 989803160611
• Wavelength rangeVI: 500 nm to 1000 nm for all specified
sensors
• Maximum optical output power: ≤ 15 mW for all specified sensors - Application Specifications
-
Device Application Specifications
Display
Display size:
4.3 inch High-resolution Touchscreen display
Resolution 480 x 272 pixels
Frequency: 50/60 Hz
Viewing Angle: ± 15Display Screen:
Waveforms,numeric values,on-screen trend data.Display waveform:
SpO2, Respiration, EtCO2Numerical data display:
SpO2, pulse rate, temperature, O2 concentration,
FiCO2, EtCO2,Communication Interfaces
• USB port (complies with the USB 2.0 standard
as a full-speed host), to
– Upgrade software
– Connect to a barcode scanner, or a serial
interface adapter
• Ethernet port, to
– Export HL7 data
– Connect the monitor to the Efficia CMS200 central station
• Wireless connectivityIII
Option E20 enables the monitor to access the EMR using
the customer’s existing wireless infrastructure. The monitor
supports the following wireless standards: IEEE802.11a, 802.11b,
802.11g, and 802.11n, operating in the 2.4 GHz or 5 GHz bands.
• EMR connectivity
– Via LAN
– Via WLANPower Supply
Integrated Power Supply,
Rated Voltage :
AC 100~240 V, 50~60 Hz,
Power Consumption :
25 VA
External DC power supply :
12V DC supplyPhysical Dimensions
Dimension (H) x (W) x (T) in mm:
152 x 82 x 30.5 mm
Weight :
450g (with clamp base)Battery
Battery Capacity :
Advanced Rechargeable Lithium Polymer battery –
7.4 V, 4000 mAh (1 Internal battery)Battery life :
Continuously operate for 6-8 hrs from Full Charge
(at a rate of 5 ml/h at 30°C under Normal Conditions)Battery Recharge :
When AC Power is ON,
Battery will Automatically Recharge
(about 5 hrs to 90% charge ),
When the pump is off,
the charging time is not longer than 4 hrsExternal Fuse
1.5 A / 250 V (User Replaceable)
Environmental Conditions
Operation Conditions:
Ambient temperature: + 5 to + 40°C,
Atmosphere Pressure: 86 ~106 kPa,
Relative humidity: ≤ 80%Storage Conditions :
Ambient temperature: – 20 to +55°C,
Atmosphere Pressure: 50 ~106 kPa,
Relative humidity: ≤ 95%Mechanical shock
Complies with mechanical shock requirement according to ISO 9919/IEC 80601-2-61 standards, for use within the healthcare facility.
Test conditions include:
• Peak acceleration: 150 m/s2 (15.3 g)
• Duration: 11 ms
• Pulse shape: Half sine
• Number of shocks: 3 shocks per direction per axis (18 total) Mechanical vibration
Complies with mechanical vibration requirement according to ISO 9919/IEC 80601-2-61 standards, for use within the healthcare facility.
Test conditions include:
• Frequency range: 10 Hz to 2000 Hz
• Resolution: 10 Hz
• Acceleration amplitude:
– 10 Hz to 100 Hz: 1.0 (m/s2)2/Hz
– 100 Hz to 200 Hz: -3.0 dB/octave
– 200 Hz to 2000 Hz: 0.5 (m/s2)2/Hz
• Duration: 10 minutes per each perpendicular axis (3 total)
Humidity
• Operating: 15% to 90% RH, non-condensing
• Storage: 15% to 90% RH, non-condensing - Safety Standards and Compliance
-
Safety Standards and Compliance
Classification & Electrical Safety Protection
Product Classification
According to the MDD 93/42/EEC Appendix Ⅸ Classification Rule 10: class II bType of protection against electric shock
Class I, equipment contains an internal powered equipmentLevel of protection against electric shock
Application of CF-type non-defibrillation discharging effect protectionEMC type
Group 1, Class AAnti-electroshock degree
ECG (RESP), TEMP, IBP, C.O., Quick Temp CF, SpO2, NIBP, CO2Ingress Protection
IPx1 (No protection against ingress of water)Disinfection/sterilization method
Standard methodWorking system
Continuous operation equipmentPower supply way
Grid power plug, removable and soft wire power supplyVisual Alarm Indication
Visual Alarms indicates with two priority colors:
Red (High Priority) and
Yellow (Low and Mid Priority),
meets according to the IEC 60601-1-8 standard requirements.Safety Standards
EN/IEC 60601-1
EN/IEC 60601-1-2
EN/IEC 60601-1-8
EN/IEC 60601-2-27
EN/ IEC 80601-2-30
IEC 60601-2-34
IEC 60601-2-49
EN/ISO 80601-2-55
EN/ISO 80601-2-61
EN/IEC 62366
EN/IEC 62304
EN/IEC 60601-1-6
EN/ISO 80601-2-56• Conformity: CE according to directive 93/42/EEC class IIb
• Protection class: Protection against electric shocks, Class I, internally powered equipment, per EN/IEC 60601-1
• Degree of protection: Type CF defibrillator-proof, per EN/IEC 60601-1
• IPX1 Ingress protection against vertically falling water drops, This device is not designed for outdoor use
• Protection against hazards of ignition of flammable anesthetic mixtures:
Equipment is not suitable for use in the presence of a flammable anesthetic mixtures with air or oxygen or nitrous
oxide, per IEC 60601-1
Additional requirements: EN 1060-1, EN 1060-3 and EN 12470-4Audio Alarm Volume :
Support multi-level volume functions
with the sound pressure range from 59-74 dB(A)
and key pad beep;
The alarm sound meets according to
the IEC 60601-1-8 standard requirements. - General Specifications
-
General Specifications
Display
Display size:
8″ High-resolution Touchscreen display
Resolution 800 x 600 pixels
Frequency: 50/60 Hz
Viewing Angle: ± 15Maximum number of waveform display:
7 tracesDisplay waveform:
ECG (up to 3 leads), Respiration, IBP,
SpO2, pulse wave, CO2,Numerical data display:
Heart rate, VPC rate, ST level,
respiration rate, IBP (systolic, diastolic,
mean), NIBP (systolic, diastolic, MAP),
SpO2, pulse rate, temperature, CO,
cardiac index, injectate temperature,
blood temperature, O2 concentration,
FiCO2, EtCO2,Communication Interfaces
• USB port (complies with the USB 2.0 standard
as a full-speed host), to
– Upgrade software
– Connect to a barcode scanner, or a serial
interface adapter
• Ethernet port, to
– Export HL7 data
– Connect the monitor to the Efficia CMS200 central station
• Wireless connectivityIII
Option E20 enables the monitor to access the EMR using
the customer’s existing wireless infrastructure. The monitor
supports the following wireless standards: IEEE802.11a, 802.11b,
802.11g, and 802.11n, operating in the 2.4 GHz or 5 GHz bands.
• EMR connectivity
– Via LAN
– Via WLANPower Supply
Integrated Power Supply,
Rated Voltage :
AC 100~240 V, 50~60 Hz,
Power Consumption :
25 VA
External DC power supply :
12V DC supplyPhysical Dimensions
Dimension (L) x (W) x (H) in mm:
260 x 138 x 245
Weight :
3.7 kg (with clamp base)Battery
Battery Capacity :
Advanced Rechargeable Lithium Polymer battery –
7.4 V, 4000 mAh (1 Internal battery)Battery life :
Continuously operate for 6-8 hrs from Full Charge
(at a rate of 5 ml/h at 30°C under Normal Conditions)Battery Recharge :
When AC Power is ON,
Battery will Automatically Recharge
(about 5 hrs to 90% charge ),
When the pump is off,
the charging time is not longer than 4 hrsExternal Fuse
1.5 A / 250 V (User Replaceable)
Environmental Conditions
Operation Conditions:
Ambient temperature: + 5 to + 40°C,
Atmosphere Pressure: 86 ~106 kPa,
Relative humidity: ≤ 80%Storage Conditions :
Ambient temperature: – 20 to +55°C,
Atmosphere Pressure: 50 ~106 kPa,
Relative humidity: ≤ 95%Mechanical shock
Complies with mechanical shock requirement according to ISO 9919/IEC 80601-2-61 standards, for use within the healthcare facility.
Test conditions include:
• Peak acceleration: 150 m/s2 (15.3 g)
• Duration: 11 ms
• Pulse shape: Half sine
• Number of shocks: 3 shocks per direction per axis (18 total) Mechanical vibration
Complies with mechanical vibration requirement according to ISO 9919/IEC 80601-2-61 standards, for use within the healthcare facility.
Test conditions include:
• Frequency range: 10 Hz to 2000 Hz
• Resolution: 10 Hz
• Acceleration amplitude:
– 10 Hz to 100 Hz: 1.0 (m/s2)2/Hz
– 100 Hz to 200 Hz: -3.0 dB/octave
– 200 Hz to 2000 Hz: 0.5 (m/s2)2/Hz
• Duration: 10 minutes per each perpendicular axis (3 total)
Humidity
• Operating: 15% to 90% RH, non-condensing
• Storage: 15% to 90% RH, non-condensing - Configuration & Optional Features
-
Configuration and Optional Features
Event Log Review
Approximately one year event log of normal use and
Min. 2000 records with time and dateMounting options
The monitor has the following mounting options:
• Roll stand
• Roll stand mounting kit
• Wall mount
• Wall channel
• Bedrail hookWireless LAN (Optional)
Wireless Data Transmission compatible with Patient Data Management Systems (Optional)
Nurse Staff call (Optional)
Max. 24 V / 0,5 A / 24 VA
Related Products
Request a quick quote
If you have request of our products or you are interested in any other medical products,
please leave messages below and we’ll reply you soon as possible within 24 hours.